Extraordinary BioDIP Seminar: Siegfried Weisenburger (Rockefeller University New York)
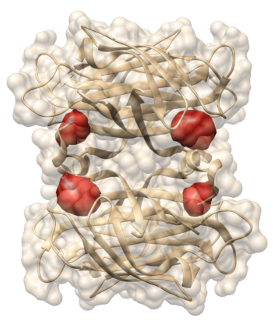
Cryogenic Optical Localization in 3D enables determination of conformational states of the cytosolic Per-ARNT-Sim domain from the histidine kinase CitA of Geobacillus thermodenitrificans, and resolve the four sites where biotin binds to streptavidin in three dimensions.
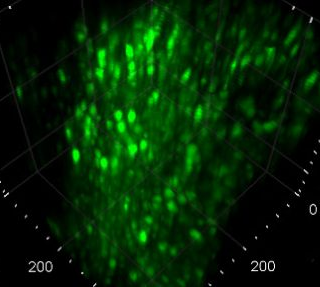
High-speed large-scale calcium imaging of neuronal networks in the mouse brain.
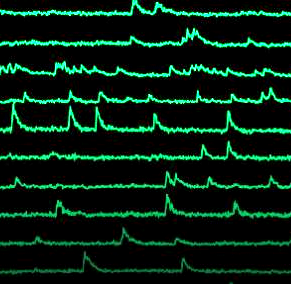
Calcium signals of neuronal networks in the mouse brain measured by Light Sculpting Microscopy.
We are happy to host Siegfried Weisenburger (Rockefeller University New York) who has published two Nature Methods papers since 2016.
The talk takes place on June 9th, 10am in the CRTD lecture hall right.
This Extraordinary Spring Seminar is dedicated to two imaging technologies:
- Cryogenic Optical Localization in 3D (COLD) which reaches Angstrom resolution in deciphering the positions of several fluorescent sites within a single small protein and
- Light Sculpting Microscopy used for high speed large-volume Ca imaging of neural networks in e.g. mouse brain.
In the first part "Cryogenic Optical Localization in 3D" (COLD) will be introduced, which reaches Angstrom resolution in deciphering the positions of several fluorescent sites within a single small protein. The key mechanism is the enhanced photostability at low temperatures, thus providing a much higher signal-to-noise ratio than in room temperature super-resolution microscopy. It will be shown how COLD can determine the conformational state of the cytosolic Per-ARNT-Sim domain from the histidine kinase CitA of Geobacillus thermodenitrificans, and resolve the four sites where biotin binds to streptavidin in three dimensions. COLD opens new doors for obtaining quantitative structure information from small to medium sized biomolecules at the Angstrom scale and can complement other existing techniques such as x-ray crystallography or magnetic resonance spectroscopy.
In the second part, the technique coined Light Sculpting Microscopy will be introduced. This method is based on temporal focusing and allows an effective decoupling of the axial and lateral confinement of the excitation volume combined with the advantage of high penetration depth and low scattering of two-photon excitation. The ability to tailor the three-dimensional spatial distribution of light within biological samples allows to maintain optical sectioning while introducing parallelization of excitation and thereby increased acquisition rates. In vivo applications of this method for high-speed large-scale calcium imaging of neuronal networks in different model organisms including the mouse brain will be presented. Light Sculpting Microscopy pushes the frontier in neuroscience and enables the interrogation of neuronal dynamics of large populations in awake, behaving animals in real time in order to unravel the complex interactions of neurons that underlie cognitive function and behavior.
